mRNA platform technologies are used to express therapeutic proteins and vaccine antigens
Our lipid nanoparticles deliver custom-designed mRNA directly to cells. Utilizing the natural translation mechanisms within the cell, Immorna’s mRNAs transform the cell into a biosynthetic factory for the target protein. This technology can be applied to generating therapeutic proteins and vaccine antigens for a broad range of indications. Our proprietary platform technologies facilitate rapid drug candidate screening and allow us to assess multiple drug candidates simultaneously, thereby reducing the timeline and cost of preclinical development.
Drug development approach based on complementary mRNA platforms
Most recently, therapeutic conventional mRNAs have demonstrated proof of concept through the large-scale use of mRNA-based COVID-19 vaccinations. The rapid adoption of mRNA-based vaccines has quickly demonstrated not only the safety and efficacy of mRNA therapies but has also highlighted their potential for dynamic development. However, at Immorna we believe there is much more to discover. Our diverse platforms allow us to further develop and explore the therapeutic potential of mRNA-based therapies. In addition to mature conventional mRNA platforms for the development of a broad range of therapeutics and vaccines, we have also optimized self-replicating RNA (srRNA) platforms tailored to multiple immuno-oncology and infectious disease applications, as appropriate. We are also exploring the use of circular RNA platforms for next-generation mRNA modalities.
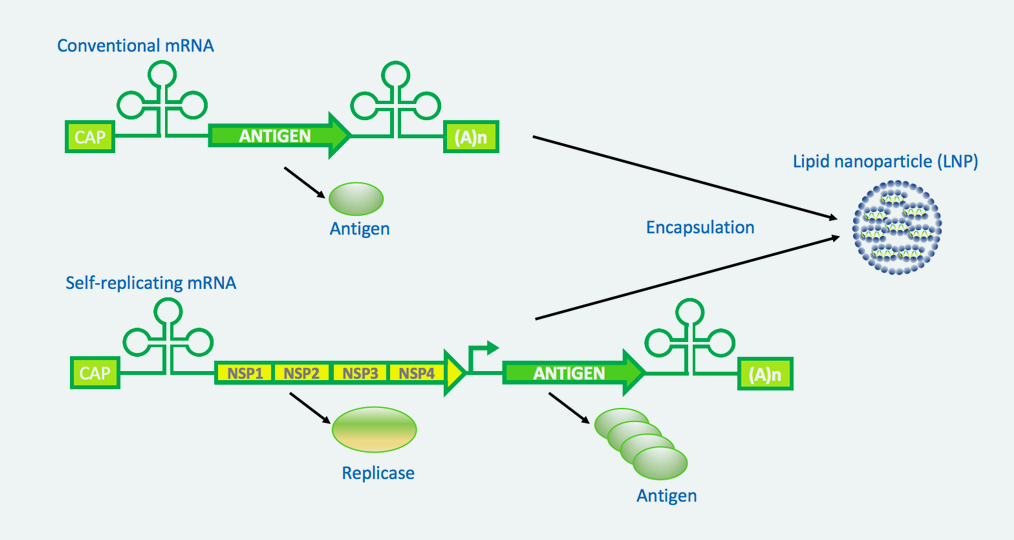

Rapid development of next generation vaccines and therapeutics based on platform mRNA technologies
Immorna has invested in creating a robust, standardized, and scalable Chemistry, Manufacturing, and Controls (CMC) platform. By utilizing readily accessible, low-cost starting materials, we can quickly launch into the development of an mRNA of interest. Our propriety formulations support enhanced final drug product stability, helping to overcome cold-chain storage bottlenecks that limit the product shelf-life and vaccine distribution.
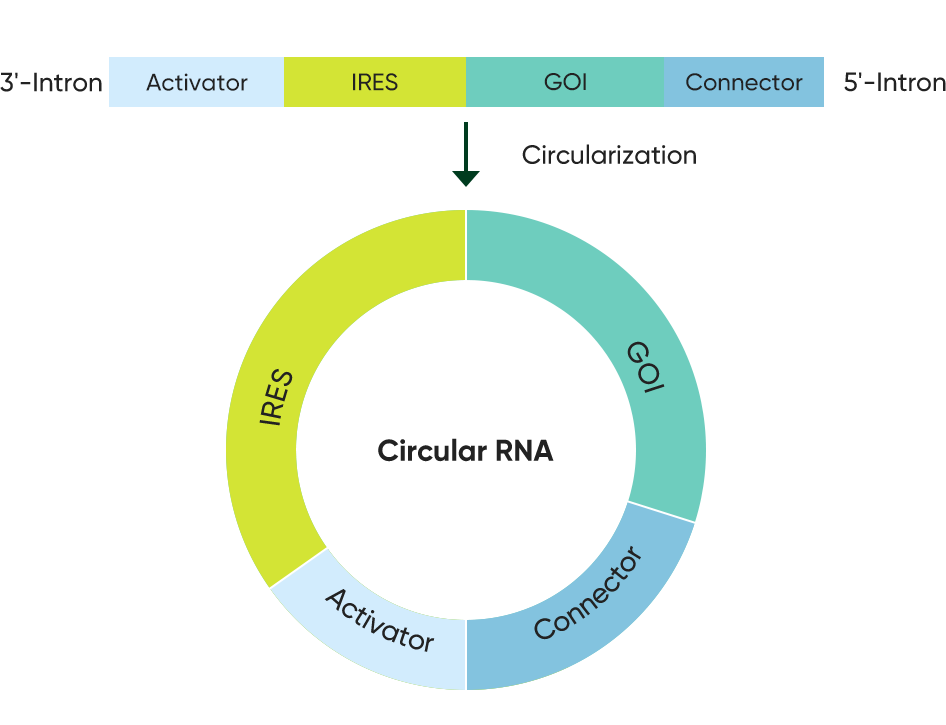
Circular mRNA
Enhanced stability and cell type-specific expression are key drivers behind Immorna’s circular RNA R&D program. Circular RNA approaches enable breakthrough RNA therapies for applications that require both extended gene expression window and tight control of immunostimulatory effects associated with RNA delivery.