Contract Development & Manufacturing Services
Immorna offers one-stop-shop contract development & manufacturing (CDMO) services, based on our proprietary RNA and LNP platforms. Development and manufacturing using customers’ unique platforms is also available after technology transfer.
Services span from sequence design, customized analytical assay development/qualification, DNA template manufacturing, mRNA/LNP production and purification, to fill-finish and QC release.
Integrated analytical and stability services support rapid transition of development programs from pre-clinical to clinical stage.
Strength in Development
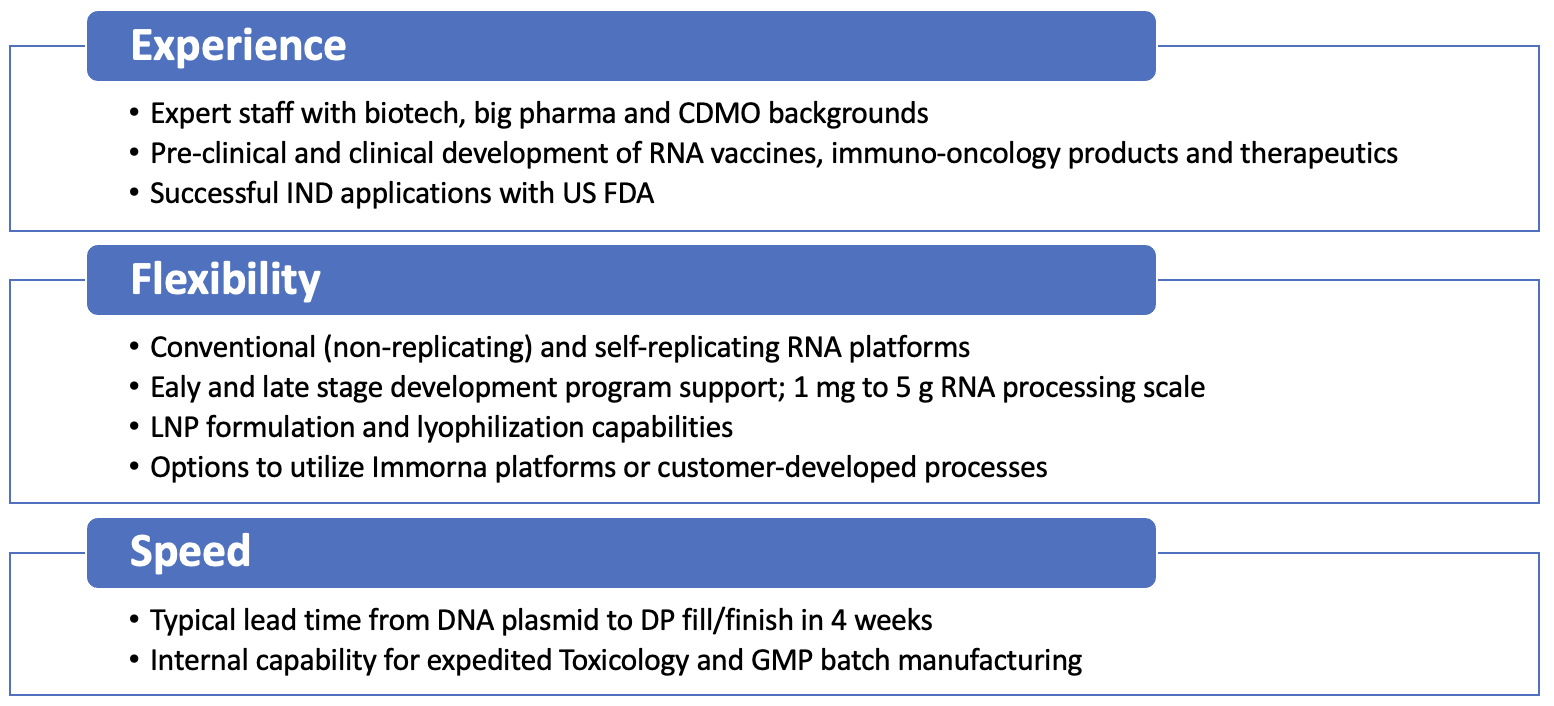
Strength in Manufacturing

Services Offered
Proprietary conventional and self-replicating RNA and LNP platforms
Customized process development
Research grade material production
Toxicology study material manufacturing and release
Clinical trial material (CTM) manufacturing and release
GMP and developmental stability studies and testing
Customized analytical assays (both release and characterization), development and qualification
IND filing support
<Insert form to get in contact with us>